Soncin Lab
- Projects
- The Team
- Publications
The Soncin lab investigates multiple aspects related to trophoblast stem and progenitor cells in the early stages of placenta. We use trophoblast primary cells and in vitro models to identify how the transcriptional signature of trophoblast cells is established and maintained. We study the epigenomic factors that regulate trophoblast specification, including transcription factors and the cell-specific epigenetic landscape they operate in. We also study the signaling pathways involved in trophoblast specification and maintenance and how external factors can influence trophoblast stem cell function. We also have projects related to comparative placentation to investigate conserved and divergent mechanisms of trophoblast stem cell specification and maintenance among non-traditional animal models.
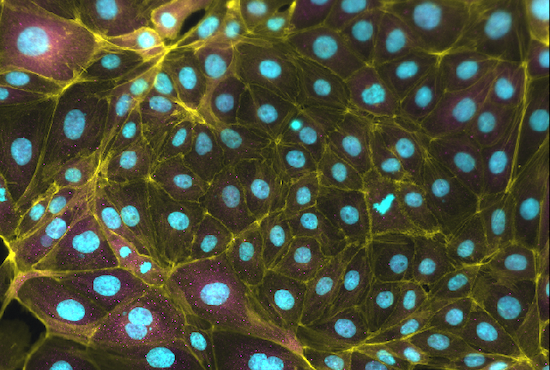
During her post-doctoral work on comparative placentation between mouse and human, Dr. Soncin identified VGLL1 as a human-specific and placenta-specific gene expressed in the trophoblast progenitor compartment of the placenta. VGLL1 is a transcription co-factor binding TEAD family members, known to be master regulator of cell-specific transcription. The complex Tead4/Yap1 is a well know regulator of trophoblast lineage specification in mouse. VGLL1 binds TEAD4 in a competitive manner to YAP1, potentially regulating a human-specific trophoblast transcriptional program. Projects funded by NIH/NICHD are currently investigating the role of VGLL1 in trophoblast stem cells and pluripotent stem cell models of trophoblast specification, and its interaction with both the HIPPO and canonical WNT pathways.
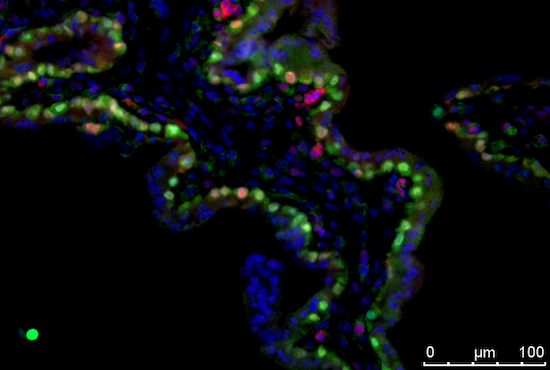
While the transcriptional program of the human trophoblast lineage has been characterized both in vivo and in vitro, little we know about the cell-type specific epigenomics landscape driving trophoblast lineage specification in human trophoblast stem/progenitor cells. The lab was recently awarded a grant from CIRM to study the mechanisms that drive trophoblast stem/progenitor cell identity in the early post-implantation human placenta. HIRING!
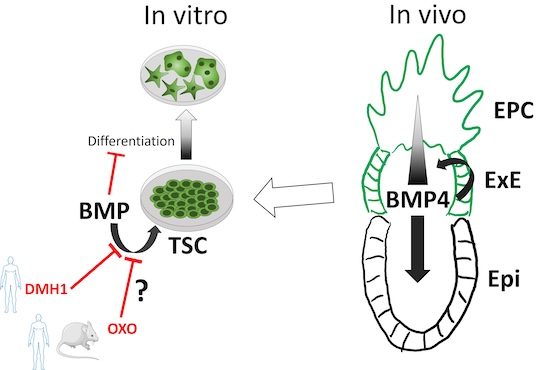
The Bone Morphogenetic Protein (BMP) signaling pathway has been implicated in the initiation of gastrulation and mesoderm specification in the developing embryo. However, increasing evidence suggest a role in the extra-embryonic compartment. We recently demonstrated that BMP signaling is active in both mouse and human trophoblast stem cells and its downregulation is required for proper differentiation into mature trophoblast cell types. As this seems to be a conserved mechanism, we are currently investigating the role of BMP signaling pathway in the developing and mature placenta using mouse models.
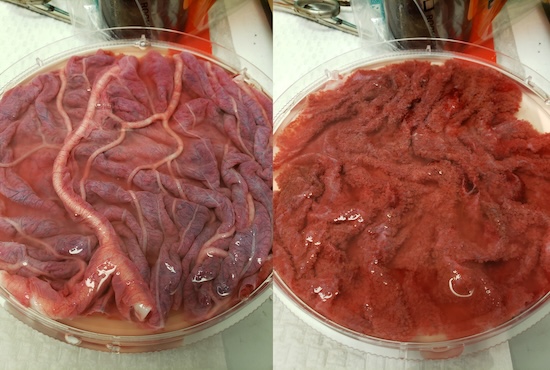
Increasing evidence suggest that defects in the trophectoderm compartment of blastocyst from artificial reproductive technologies (ART) could be one of the causes of higher incidence of pregnancy losses associated with ART. One of the strategies to “Save the white rhino” is the use of ART to obtain live birth of white rhino embryos in surrogate mothers. Using rhino iPSC derived in the laboratories of the San Diego Zoo Wildlife Alliance and in vitro models developed in our lab, we are studying the mechanisms of trophoblast lineage specification in rhinos. In collaboration with the Reproductive Department of the SDZWA, we also have collected three term rhino placentas, and we are optimizing tools for cellular and molecular biology assays.